HPV vaccination: How the world can eliminate cervical cancer
HPV vaccines offer a rare opportunity to effectively eliminate one type of cancer. By taking this opportunity, it’s possible to save hundreds of thousands of women each year.
Every year, over half a million women develop cervical cancer, and more than 300,000 die from the disease. These deaths are particularly tragic because we can prevent them through vaccination and early screening.
It’s possible to virtually eliminate this type of cancer with existing vaccines. Some countries are already on track to achieve this goal within a decade, but many others lag behind.
This is a huge opportunity to save hundreds of thousands of women every year.
In this article, we describe the cause of cervical cancer and how we can use incredibly effective vaccines to eliminate it.
Cervical cancer is caused by a virus, which makes it preventable
Cervical cancer is one of several cancers caused by pathogens — in this case, the “human papillomavirus” (HPV).
There are hundreds of types of the virus, but only a handful are responsible for most cases of cervical cancer.
The virus is also responsible for a large share of other cancers, including anal cancer, penile cancer, vulval cancer, vaginal cancer, and some head and neck cancers.1
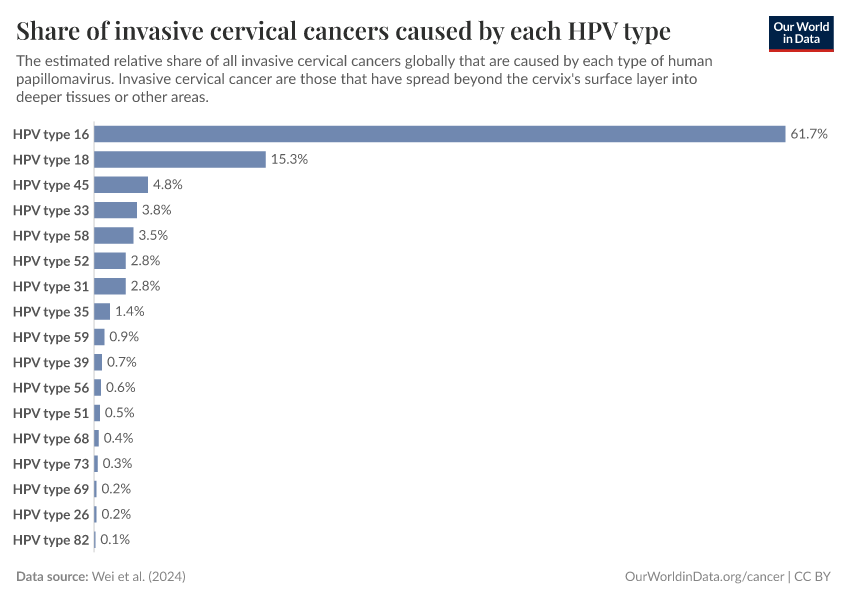
What share of invasive cervical cancers are caused by each type of the human papillomavirus?
See the data in our interactive visualization

What share of different cancers are caused by the human papillomavirus?
See the data in our interactive visualization
How does the virus cause these cancers?
HPV is a very common virus that spreads through physical contact — especially through sex, kissing, or touching — and can infect cells in the cervix, vagina, penis, mouth, and some other parts of the body.
In some people infected by HPV, the virus integrates itself into cells’ DNA and damages key proteins that protect us from uncontrolled cell growth — eventually leading to cancer.
This link between HPV and cervical cancer was uncovered in the 1980s by the scientist Harald zur Hausen, who later won a Nobel prize for the discovery.2
Before this, researchers debated whether hormones, environmental toxins, or other factors were responsible. Zur Hausen’s team focused on human papillomaviruses, which were then known to cause genital warts.
The team identified viral DNA in cervical cancer cells and found that the virus was present in the cancer cells and integrated their DNA. HPV was able to disrupt key functions of cells by damaging proteins like “p53”, which is critical in stopping cells from multiplying if they develop potentially harmful mutations.3
Further research confirmed their findings and helped uncover the role of HPV in a range of other cancers.1
HPV vaccines are highly effective in preventing infections and cervical cancer
Because cervical cancer is caused by a virus, it can be prevented through vaccination.
The first HPV vaccine was developed in the 1990s by researchers in Australia.4
It was first introduced in 2006 and is effective against four major types of HPV.5 Another version, introduced in 2014, expanded its coverage to protect against additional cancer-causing HPV types.6
These vaccines work by stimulating the immune system to produce antibodies that can eliminate HPV and prevent infections.7
HPV vaccines are highly effective, which has been demonstrated in large-scale randomized controlled trials. Research shows that, if given early, vaccination reduces the risk of serious cervical cell changes by 99% for the HPV types most likely to cause cervical cancer.8
Recent research has found that even a single dose provides high efficacy against infections.9
The vaccine is most effective when given early — before people are exposed to the virus. Vaccination programs in schools are, therefore, very impactful in making the most of HPV vaccines.
There is also another important reason for giving out these vaccines in schools: the vaccine’s efficacy is long-lasting10, and vaccination programs are much easier to implement at scale through schools than vaccinating individuals at clinics.11
Cervical cancer rates have declined greatly among younger generations with vaccination
In England, younger cohorts vaccinated at school show dramatically lower rates of cervical cancer than older cohorts when they reached the same age. This is shown in the chart below.12
In the youngest cohort, which had a vaccination rate of 89%, cervical cancer rates were around 87% lower than in the oldest cohorts.
Large reductions in cervical cancer rates have also been seen in other countries with high vaccination rates.13
Because of highly effective vaccination and early screening programs, several countries have substantially reduced cervical cancer rates.
Rates in Australia and the United Kingdom, for example, have recently fallen below 6 per 100,000 and are projected to fall below 4 per 100,000 in the coming decade.14
But, at current vaccination rates, it will take decades for other countries to reach these levels — this is why raising vaccination rates is crucial.15
Hundreds of thousands of women still get cervical cancer each year
Cervical cancer still affects hundreds of thousands of women globally, despite the fact that vaccines and screening are highly effective. Screening and diagnosis can inform surgeries that could cure cervical cancer if identified in its early stages.
It’s estimated that there were more than 660,000 new cases of cervical cancer and around 350,000 deaths globally in 2022.16
The map shows that cervical cancer is much more common in Africa and South America than in other regions.

How common is cervical cancer screening within countries?
Explore cervical cancer screening rates in European countries
There are two main reasons that cervical cancer deaths remain high.
One reason is that women in many poor countries lack access to early screening, as shown in the map above. Another is that women living with HIV, which is much more common in sub-Saharan Africa, are much more at risk of cervical cancer because their immune system is weakened.17
The final reason is that HPV vaccination rates are low or vaccines are unavailable in many countries.
HPV vaccination is limited in many countries
In many countries in Africa and Southeast Asia, vaccination rates are low. This is shown in the chart below. In many countries, vaccination rates are very low.
However, this is not true everywhere in these regions. Ethiopia and Rwanda, for example, have had relatively high vaccination rates in recent years due to targeted campaigns.
This is visible in the second map, which shows the national policy for offering HPV vaccines. In the countries shown in blue — much of the Americas, Europe, and Australia — HPV vaccines are offered routinely at no cost at a national level.
But in many other countries across Africa and Asia, shown in red, they are not.
In China, for example, the HPV vaccine has only recently been offered in certain cities and regions, but not across the country, and the national vaccination rate is very low.18
Another example is Japan, where the HPV vaccine had been withdrawn for several years (between 2013 and 2021) because of inaccurate media reports that the vaccine was linked to pregnancy complications.19 Large-scale studies have shown no difference in side effects in those taking the HPV vaccine20, and the vaccine has been recently reintroduced.
Finally, many countries have low vaccination rates due to limited supplies and the upfront costs of bulk buying the vaccine, which have led many to delay its introduction. This is especially true in middle-income countries that are not eligible for funding through Gavi, which helps improve access to vaccines in low-income countries.21
Recently, new HPV vaccines have been developed, which are expected to be cheaper to manufacture, but they are not yet available internationally.22 These could increase the global supply, help improve vaccination rates in lower- and middle-income countries, and help push the elimination of cervical cancer globally.
The world is missing out on the opportunity to prevent millions of cervical cancer cases
Countries like the UK and Australia are showing that the elimination of cervical cancer is possible, but much of the world is missing out.
Higher vaccination rates could prevent hundreds of thousands of cervical cancer cases every year.
This is shown in the chart below, which presents projections by Kate T. Simms and colleagues based on estimates of current cases, vaccination, and screening rates worldwide and modeling of the transmission of HPV and the vaccine’s effectiveness.23
They estimate that 80% to 100% vaccination rates for both boys and girls, with catch-up vaccinations for adults, could prevent nearly 50 million cervical cancer cases by 2100.
Countries with widespread HPV vaccination and regular cervical cancer screenings are already seeing dramatic reductions in cancer rates. They demonstrate that it’s possible to effectively eliminate the disease.
Yet many countries still lag behind. Some face barriers in accessing limited supplies of vaccines, while others have had low vaccination rates due to popular misconceptions.
However, HPV vaccines offer a rare opportunity to effectively eliminate a common type of cancer.
By investing in vaccination and screening, the world could save hundreds of thousands of lives each year, prevent avoidable suffering, and make cervical cancer a disease of the past.
Endnotes
De Sanjosé, S., Serrano, B., Tous, S., Alejo, M., Lloveras, B., Quirós, B., Clavero, O., Vidal, A., Ferrándiz-Pulido, C., Pavón, M. Á., Holzinger, D., Halec, G., Tommasino, M., Quint, W., Pawlita, M., Muñoz, N., Bosch, F. X., Alemany, L., RIS HPV TT, VVAP and Head and Neck study groups, & Kulkarni, A. (2018). Burden of Human Papillomavirus (HPV)-Related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectrum, 2(4), pky045. https://doi.org/10.1093/jncics/pky045
Schiffman, M., Doorbar, J., Wentzensen, N., De Sanjosé, S., Fakhry, C., Monk, B. J., Stanley, M. A., & Franceschi, S. (2016). Carcinogenic human papillomavirus infection. Nature Reviews Disease Primers, 2(1), 16086. https://doi.org/10.1038/nrdp.2016.86
zur Hausen, H. (2009). The search for infectious causes of human cancers: where and why (Nobel lecture). Angewandte Chemie International Edition, 48(32), 5798-5808. https://doi.org/10.1002/anie.200901917
Zur Hausen, H. (2009). Papillomaviruses in the causation of human cancers—A brief historical account. Virology, 384(2), 260–265. https://doi.org/10.1016/j.virol.2008.11.046
Frazer, I. H. (2019). The HPV Vaccine Story. ACS Pharmacology & Translational Science, 2(3), 210–212. https://doi.org/10.1021/acsptsci.9b00032
Specifically, it is effective against types 6, 11, 16, and 18.
Markowitz, L. E., & Schiller, J. T. (2021). Human Papillomavirus Vaccines. The Journal of Infectious Diseases, 224(Supplement_4), S367–S378. https://doi.org/10.1093/infdis/jiaa621
Markowitz, L. E., & Schiller, J. T. (2021). Human Papillomavirus Vaccines. The Journal of Infectious Diseases, 224(Supplement_4), S367–S378. https://doi.org/10.1093/infdis/jiaa621
These efficacy estimates are shown in the results of Analysis 1.1 -
Arbyn, M., Xu, L., Simoens, C., & Martin-Hirsch, P. P. (2018). Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database of Systematic Reviews, 2020(3). https://doi.org/10.1002/14651858.CD009069.pub3
International Agency for Research on Cancer. (2023). Protection from a Single Dose of HPV Vaccine. Available online.
Basu, P., Malvi, S. G., Joshi, S., Bhatla, N., Muwonge, R., Lucas, E., Verma, Y., Esmy, P. O., Poli, U. R. R., Shah, A., Zomawia, E., Pimple, S., Jayant, K., Hingmire, S., Chiwate, A., Divate, U., Vashist, S., Mishra, G., Jadhav, R., … Sankaranarayanan, R. (2021). Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. The Lancet Oncology, 22(11), 1518–1529. https://doi.org/10.1016/S1470-2045(21)00453-8
Basu, P., Malvi, S. G., Joshi, S., Bhatla, N., Muwonge, R., Lucas, E., Verma, Y., Esmy, P. O., Poli, U. R. R., Shah, A., Zomawia, E., Pimple, S., Jayant, K., Hingmire, S., Chiwate, A., Divate, U., Vashist, S., Mishra, G., Jadhav, R., … Sankaranarayanan, R. (2021). Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. The Lancet Oncology, 22(11), 1518–1529. https://doi.org/10.1016/S1470-2045(21)00453-8
Ladner, J., Besson, M. H., Rodrigues, M., Audureau, E., & Saba, J. (2014). Performance of 21 HPV vaccination programs implemented in low and middle-income countries, 2009–2013. BMC public health, 14, 1-11. Available online.
Kempe, A., Allison, M. A., & Daley, M. F. (2018). Can School-Located Vaccination Have a Major Impact on Human Papillomavirus Vaccination Rates in the United States? Academic Pediatrics, 18(2), S101–S105. https://doi.org/10.1016/j.acap.2017.08.010
Falcaro, M., Castañon, A., Ndlela, B., Checchi, M., Soldan, K., Lopez-Bernal, J., Elliss-Brookes, L., & Sasieni, P. (2021). The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. The Lancet, 398(10316), 2084–2092. https://doi.org/10.1016/S0140-6736(21)02178-4
Jansen, E. E. L., Zielonke, N., Gini, A., Anttila, A., Segnan, N., Vokó, Z., Ivanuš, U., McKee, M., De Koning, H. J., De Kok, I. M. C. M., Veerus, P., Anttila, A., Heinävaara, S., Sarkeala, T., Csanádi, M., Pitter, J., Széles, G., Vokó, Z., Minozzi, S., … Priaulx, J. (2020). Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. European Journal of Cancer, 127, 207–223. https://doi.org/10.1016/j.ejca.2019.12.013
Brisson, M., Kim, J. J., Canfell, K., Drolet, M., Gingras, G., Burger, E. A., Martin, D., Simms, K. T., Bénard, É., Boily, M.-C., Sy, S., Regan, C., Keane, A., Caruana, M., Nguyen, D. T. N., Smith, M. A., Laprise, J.-F., Jit, M., Alary, M., … Hutubessy, R. (2020). Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet, 395(10224), 575–590.
Hall, M. T., Simms, K. T., Lew, J.-B., Smith, M. A., Brotherton, J. M., Saville, M., Frazer, I. H., & Canfell, K. (2019). The projected timeframe until cervical cancer elimination in Australia: A modelling study. The Lancet Public Health, 4(1), e19–e27. https://doi.org/10.1016/S2468-2667(18)30183-X
Davies-Oliveira, J. C., Smith, M. A., Grover, S., Canfell, K., & Crosbie, E. J. (2021). Eliminating Cervical Cancer: Progress and Challenges for High-income Countries. Clinical Oncology, 33(9), 550–559. https://doi.org/10.1016/j.clon.2021.06.013
Simms, K. T., Steinberg, J., Caruana, M., Smith, M. A., Lew, J.-B., Soerjomataram, I., Castle, P. E., Bray, F., & Canfell, K. (2019). Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: A modelling study. The Lancet Oncology, 20(3), 394–407. https://doi.org/10.1016/S1470-2045(18)30836-2
Canfell, K., Kim, J. J., Brisson, M., Keane, A., Simms, K. T., Caruana, M., Burger, E. A., Martin, D., Nguyen, D. T. N., Bénard, É., Sy, S., Regan, C., Drolet, M., Gingras, G., Laprise, J.-F., Torode, J., Smith, M. A., Fidarova, E., Trapani, D., … Hutubessy, R. (2020). Mortality impact of achieving WHO cervical cancer elimination targets: A comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet, 395(10224), 591–603. https://doi.org/10.1016/S0140-6736(20)30157-4
This was estimated by the WHO’s Global Cancer Observatory for 2022.
Liu, G., Sharma, M., Tan, N., & Barnabas, R. V. (2018). HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS, 32(6), 795–808. https://doi.org/10.1097/QAD.0000000000001765
Rohner, E., Bütikofer, L., Schmidlin, K., Sengayi, M., Maskew, M., Giddy, J., Taghavi, K., Moore, R. D., Goedert, J. J., Gill, M. J., Silverberg, M. J., D’Souza, G., Patel, P., Castilho, J. L., Ross, J., Sohn, A., Bani‐Sadr, F., Taylor, N., Paparizos, V., … Bohlius, J. (2020). Cervical cancer risk in women living with HIV across four continents: A multicohort study. International Journal of Cancer, 146(3), 601–609. https://doi.org/10.1002/ijc.32260
Stelzle, D., Tanaka, L. F., Lee, K. K., Ibrahim Khalil, A., Baussano, I., Shah, A. S. V., McAllister, D. A., Gottlieb, S. L., Klug, S. J., Winkler, A. S., Bray, F., Baggaley, R., Clifford, G. M., Broutet, N., & Dalal, S. (2021). Estimates of the global burden of cervical cancer associated with HIV. The Lancet Global Health, 9(2), e161–e169. https://doi.org/10.1016/S2214-109X(20)30459-9
Wang, H., Jiang, Y., Wang, Q., Lai, Y., & Holloway, A. (2023). The status and challenges of HPV vaccine programme in China: An exploration of the related policy obstacles. BMJ Global Health, 8(8), e012554. https://doi.org/10.1136/bmjgh-2023-012554
Haruyama, R., Obara, H., & Fujita, N. (2022). Japan resumes active recommendations of HPV vaccine after 8·5 years of suspension. The Lancet Oncology, 23(2), 197–198. https://doi.org/10.1016/S1470-2045(22)00002-X
Ikeda, S., Ueda, Y., Yagi, A., Matsuzaki, S., Kobayashi, E., Kimura, T., ... & Kudoh, K. (2019). HPV vaccination in Japan: what is happening in Japan?. Expert review of vaccines, 18(4), 323-325. https://www.tandfonline.com/doi/abs/10.1080/14760584.2019.1584040
Hanley, S. J., Yoshioka, E., Ito, Y., & Kishi, R. (2015). HPV vaccination crisis in Japan. The Lancet, 385(9987), 2571. https://doi.org/10.1016/S0140-6736(15)61152-7
Arbyn, M., & Xu, L. (2018). Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Review of Vaccines, 17(12), 1085–1091. https://doi.org/10.1080/14760584.2018.1548282
Faksová, K., Laksafoss, A. D., & Hviid, A. (2024). Human papillomavirus nonavalent (HPV9) vaccination and risk of immune mediated diseases, myocarditis, pericarditis, and thromboembolic outcomes in Denmark: Self-controlled case series study. BMJ Medicine, 3(1), e000854. https://doi.org/10.1136/bmjmed-2024-000854
World Health Organization. (2018). Global Market Study: HPV. Available online.
World Health Organization. (2020). Global strategy to accelerate the elimination of cervical cancer as a public health problem. Available online.
World Health Organization. (2024). Considerations for Human Papillomavirus (HPV) Vaccine Product Choice. Available online.
International Agency for Research on Cancer. (2023). Protection from a Single Dose of HPV Vaccine. Available online.
Simms, K. T., Steinberg, J., Caruana, M., Smith, M. A., Lew, J.-B., Soerjomataram, I., Castle, P. E., Bray, F., & Canfell, K. (2019). Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: A modelling study. The Lancet Oncology, 20(3), 394–407. https://doi.org/10.1016/S1470-2045(18)30836-2
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this article, please also cite the underlying data sources. This article can be cited as:
Saloni Dattani and Veronika Samborska (2024) - “HPV vaccination: How the world can eliminate cervical cancer” Published online at OurWorldinData.org. Retrieved from: 'https://ourworldindata.org/hpv-vaccination-world-can-eliminate-cervical-cancer' [Online Resource]BibTeX citation
@article{owid-hpv-vaccination-world-can-eliminate-cervical-cancer,
author = {Saloni Dattani and Veronika Samborska},
title = {HPV vaccination: How the world can eliminate cervical cancer},
journal = {Our World in Data},
year = {2024},
note = {https://ourworldindata.org/hpv-vaccination-world-can-eliminate-cervical-cancer}
}Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.



